Zettl Group Page |
Research Highlights
|

|

|

|
Imaging and Dynamics of Light Atoms and Molecules on Graphene: Supplementary materials
J.C. Meyer, C.O. Girit, M.F. Crommie, and A. ZettlDepartment of Physics, University of California at Berkeley
Materials Sciences Division, Lawrence Berkeley National Laboratory
Berkeley, CA 94720, U.S.A.
The following media files are intended for public access. If you use any of the following, you must include the credit "Courtesy Zettl Research Group, Lawrence Berkeley National Laboratory and University of California at Berkeley." All rights reserved ©2008.
Introduction
We demonstrate a means to observe, by conventional transmission electron microscopy (TEM), even the smallest atoms and molecules. Using as the TEM sample support membrane a suspended clean single layer of graphene, adsorbates such as atomic hydrogen and carbon can be seen as if they were suspended in free space. We directly image such individual adatoms, along with adsorbed carbon chains, and vacancies in the graphene itself, and investigate their real-time dynamics. These techniques facilitate observation of dynamics of more complex chemical reactions or the identification of atomic-scale structure of unknown adsorbates. In addition, the study of atomic-scale manipulation of graphene provides useful insights for graphene-based nanoelectronic applications.
Still Images
If you use any of the following images, please include the credit "Courtesy Zettl Research Group, Lawrence Berkeley National Laboratory and University of California at Berkeley."
|
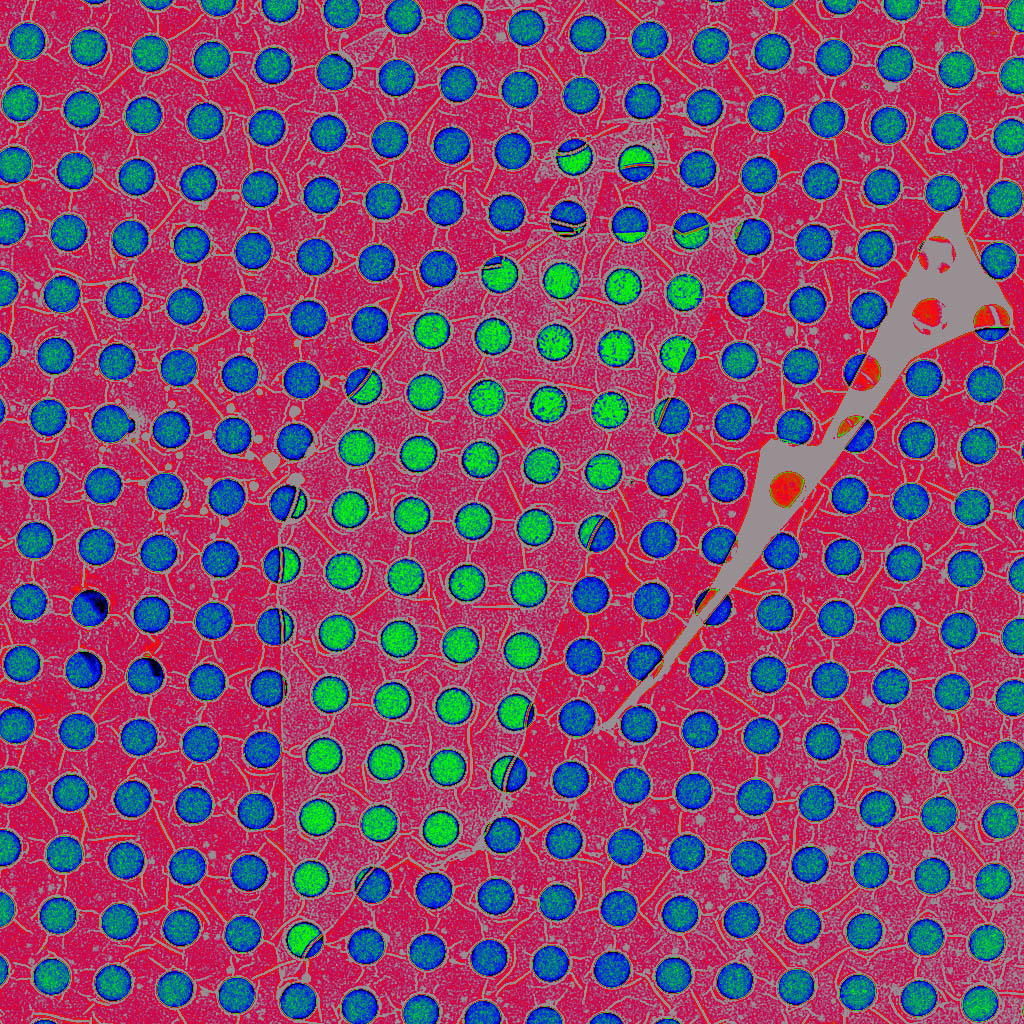
In TEM imaging, the sample under study is placed on a TEM "grid", which serves the same purpose that a microscope slide does for optical microscopy: it physically supports the sample. For high resolution TEM work, the grid is often composed of amorphous carbon with holes in it. If possible, the sample is suspended across the holes, or cantilevered out over a hole, in order to avoid background signal from the grid material when imaging electrons go through the sample. However, often it is not possible to place the sample over a hole. For example, how could a single atom be suspended over the hole? The answer is to first span the hole with a very thin membrane, like ultra-thin saran wrap spanning an open bowl. This thin membrane then supports the sample. We use a single-atom-thick graphene layer, the thinnest, strongest continuous material known, to span the holes. TEM grid with 1痠 holes. The holes with a greenish hue have been spanned with a single graphene layer. Graphene sheet (high res.) (TIFF 69.4 MB) |
|
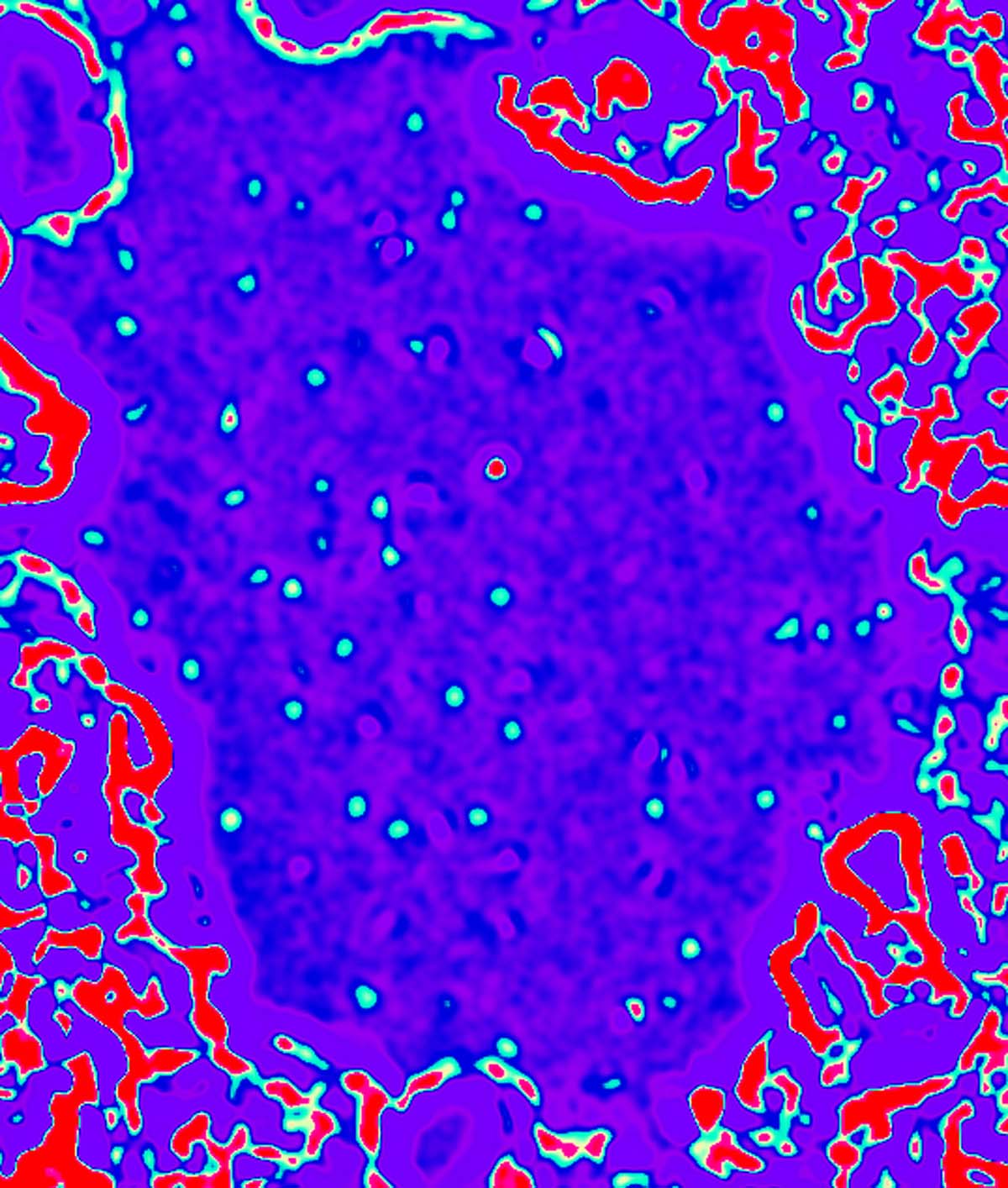
A conventional TEM does not have the resolution to "see" the individual carbon atoms in the graphene layer, as these carbon atoms are periodically spaced approximately 0.14nm apart, which is below the resolution limit of most TEMs. Hence such a membrane appears largely "invisible" in a conventional TEM (contributing only a weak uniform background signal). However, small perturbations to the film, as produced, for example, by adsorbed atoms, molecules, or missing carbon atoms in the membrane itself, are visible in the TEM. This sensitivity enhancement (not resolution enhancement) is what allows us to image individual atoms and molecules, including very light atoms such as carbon and even hydrogen. False-color TEM image of isolated hydrogen atoms (blue-green) and an isolated carbon atom (red) on a graphene membrane. Hydrogen on graphene (TIFF 4.87 MB) |
|
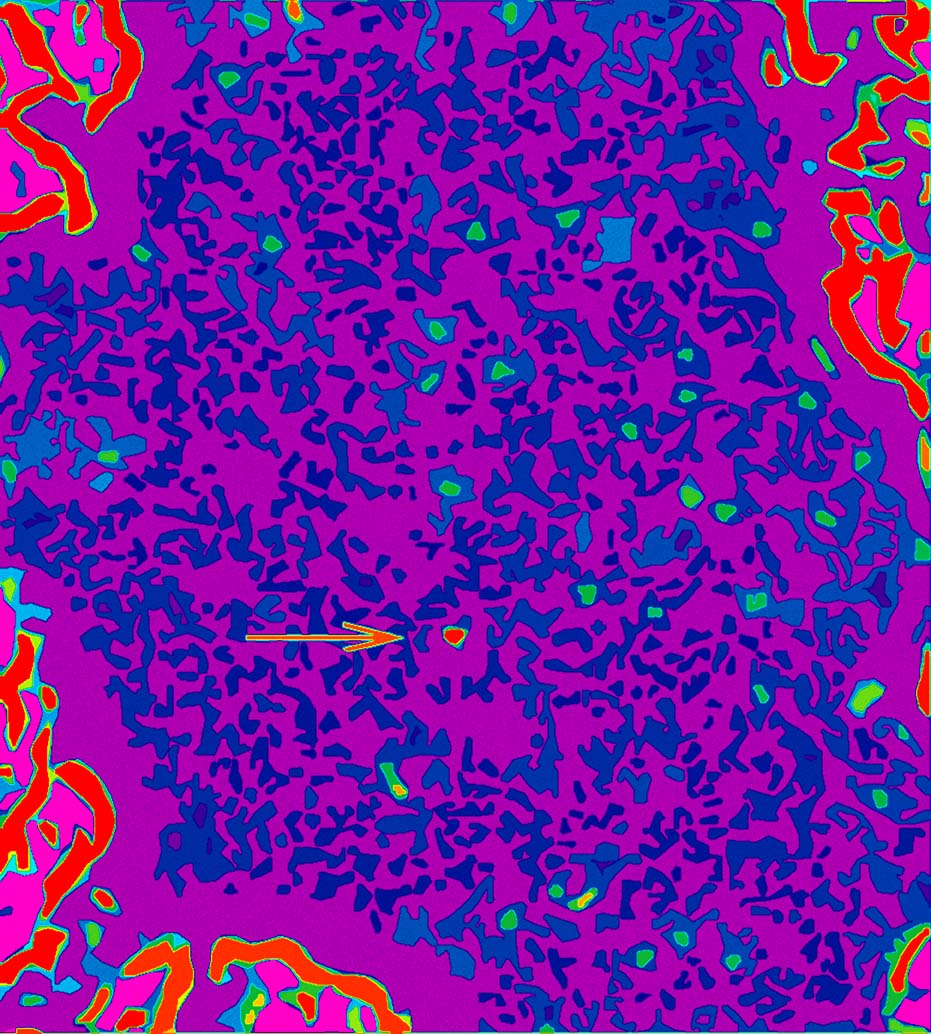
False-color TEM image of isolated hydrogen atoms (green) and an isolated carbon atom (red) on a graphene membrane Hydrogen (green) on graphene (TIFF 47.8 MB) |
|
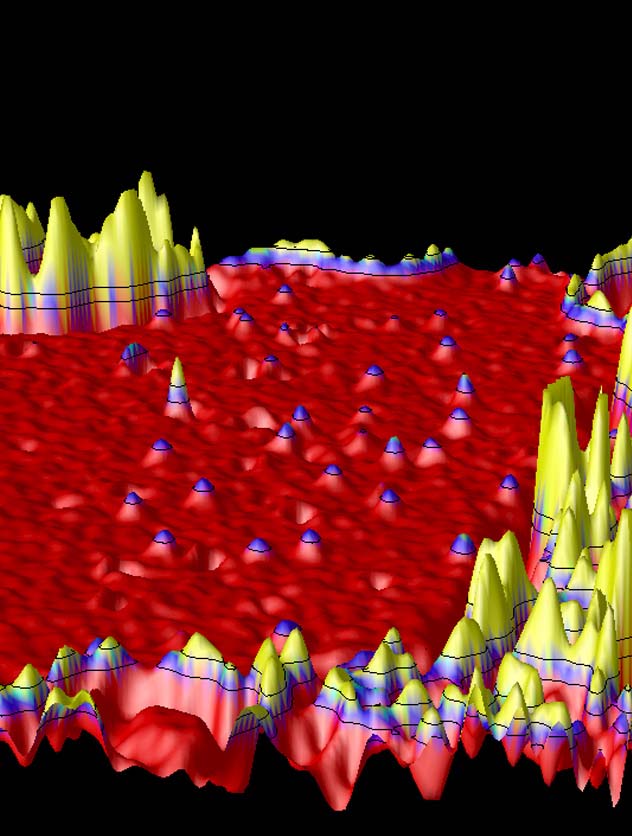
False-color 3-D rendered TEM image of isolated hydrogen atoms (purple-tipped) and an isolated carbon atom (red-tipped) on a graphene membrane. The "mountain ranges" in the fore- and background are amorphous carbon. Hydrogen on graphene, 3-D (TIFF 1.52 MB) |
Videos
If you use any of the following videos, please include the credit "Courtesy Zettl Research Group, Lawrence Berkeley National Laboratory and University of California at Berkeley."

|
Not only can we record static images atoms and molecules in the TEM, but we can also track their dynamics. This opens up the possibility of studying structural, mechanical, and electronic properties of isolated molecules, as well as the interactions between atoms and molecules (i.e. chemical reactions). Our system is also well suited for the study of selected biological specimens. The following videos show dynamics of alkane molecules on graphene. In one case the molecule moves like a catepillar, sticking and releasing from the graphene substrate. In the other case, the molecule continuously spans the distance between two rigid bodies (amorphous carbon), but changes shape. In the last few frames of the video, one end of the molecule breaks free, and the molecule "snaps back" like a rubber band. Adatoms on graphene (Quicktime 243 KB) |
Acknowledgements
This work was supported by the US Department of Energy.
External links
Nature magazine wrote a non-technical "News and Views" article on this research, which may be found here.
The original article is here.
Last modified: Wed Jul 16 15:55:20 Pacific Daylight Time 2008